Research
Mission Statement
To gain this necessary understanding we investigate well-defined model systems based on metal single crystals, oxide thin films, alloy materials, or supported metal nanoparticles. We believe in an interdisciplinary approach: Our systems are characterized with a variety of „classical“ surface science methods (like X-ray diffraction, near-ambient pressure X-ray photoemission…) coupled with gas analysis (gas chromatography, mass spectrometry…), electrochemical methods (electrochemical impedance spectroscopy…) and theoretical modelling (using density functional theory).
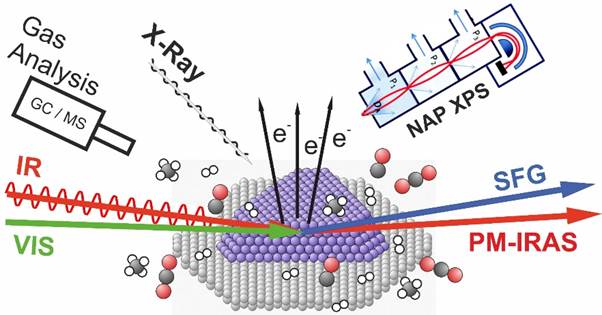
Reactions, we are interested in
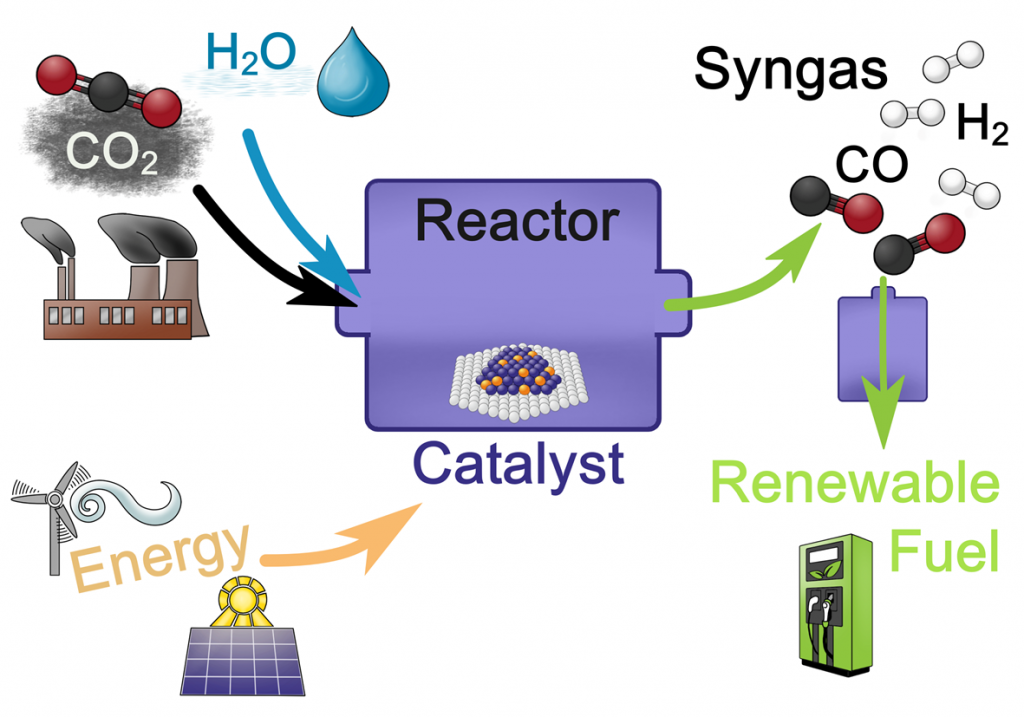
Our focus is on catalytic reactions highly relevant for renewable energy technology, chemical energy conversion and environmental protection. Examples are the utilization of the greenhouse gas CO2 and CH4 (e.g. by dry reforming or reverse water-gas shift) and their transformation into synthesis gas or renewable fuels.
These reactions are important for power to gas or power to fuel technology, which has the ability to generate a closed carbon cycle or to store excess energy from wind or solar power.
Current Projects
Tuneable Catalyst Surfaces for Heterogeneous Catalysis (ERC Grant TUCAS)
In heterogeneous catalysis, surfaces decorated with uniformly dispersed, catalytically highly active particles are a very important feature of catalysts. The goal of our ERC project is to tune and modify surface chemistry to increase catalytic performance.
Our approach to such catalyst design within the scope of this project is to use perovskite-type catalysts — for two reasons:
(i) They are a very versatile class of materials with a multitude of possible compositions and different dopings.
(ii) Under certain conditions, perovskites are capable of exsolution, which means ions of the structure (either „regular“ lattice ions or dopants) migrate to the surface and form – ideally – catalytically active clusters or nanoparticles.
Our perovskites samples are subject to extensive structural and surface morphology investigations (XRD, SEM, and simulations with DFT), and performance tests of their catalytical performance.
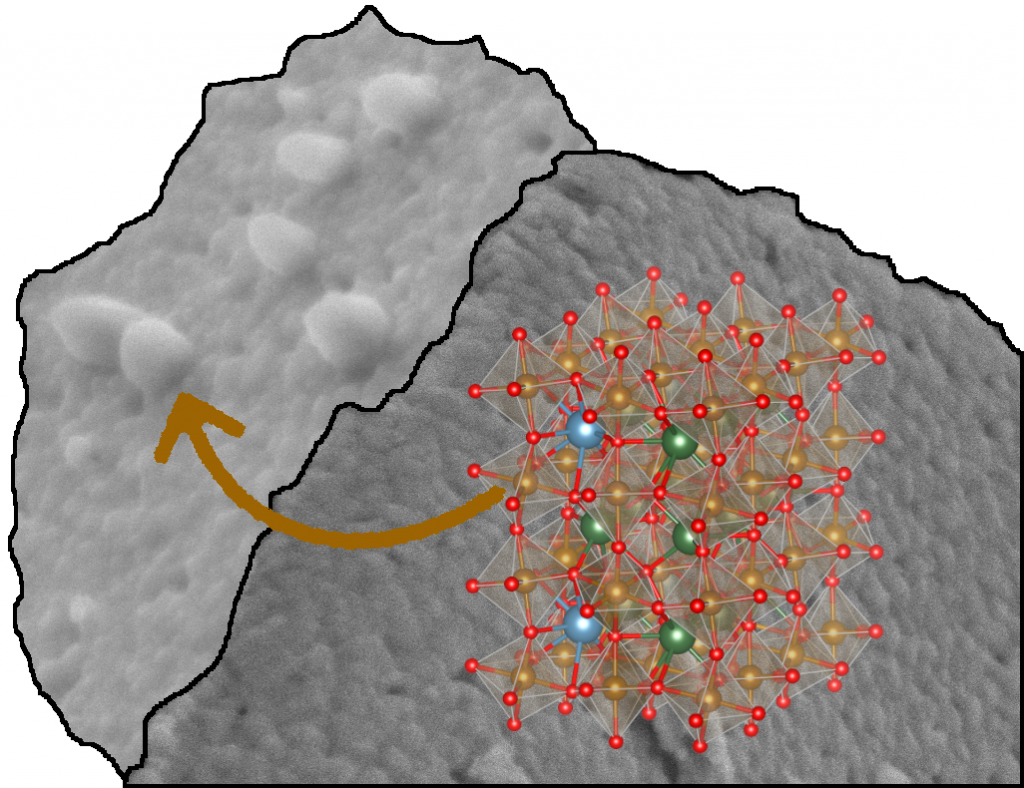
Lindenthal et al., Acta Cryst. B, 2020
Latest publications: Thomas et al. in Frontiers For Young Minds, Florian et al. in Applied Catalysis B, Thomas and Richard et al. in Fuels, Lorenz and Joel et al. in Catalysts, Lorenz et al. in Applied Catalysis B, Thomas et al. in Frontiers For Young Minds, Lorenz et al. in Acta Crystallographica B
Design of in-situ Spectroscopic Equipment
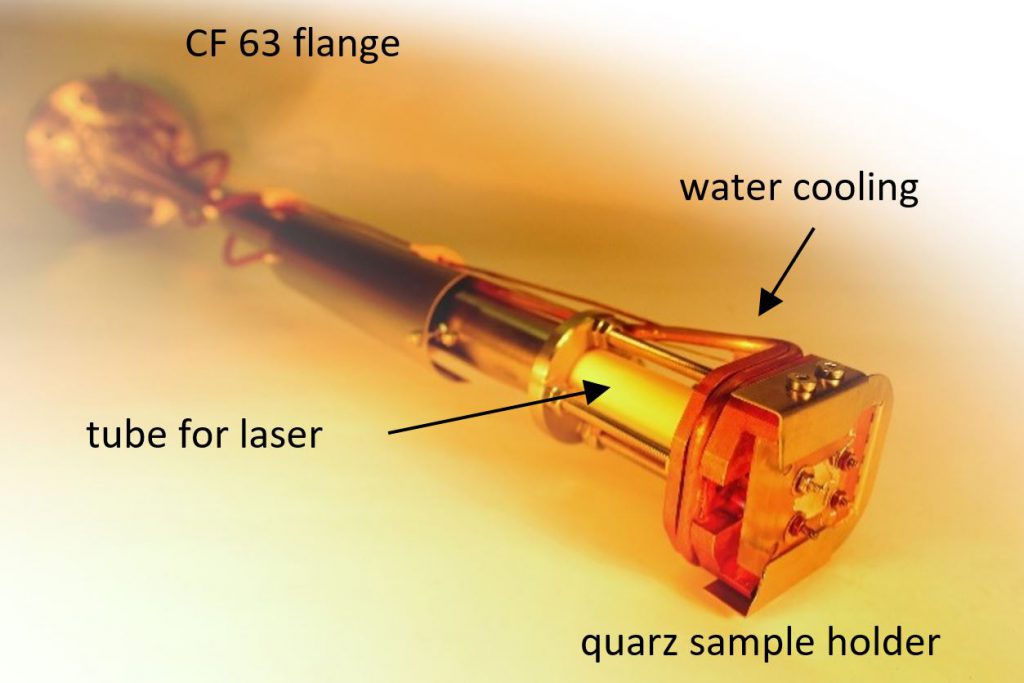
To understand catalytic processes, intensive characterization and investigation of the catalyst under operating conditions (in-situ or operando) is key. Therefore, the design and development of equipment is an ongoing endeavour in our group.
A recent example for our achievments in this area is the development of a novel sample stage for lab-based NAP-XPS, allowing us to simultaneously probe surfaces (with the surface-sensitive XPS technique) and investigate dielectric bulk properties (with EIS).
Latest publications: Raffael et al. in Crystals
CO2 Activation - Understanding Elementary Reaction Steps for Better Catalysts
In our project on CO2 activation, we investigate the interaction of gas molecules with different surfaces for utilizing CO2 as sustainable carbon source.
Due to the stable nature of CO2, it requires its activation by catalytically active oxides on which CO2 can form different surface bound carbonaceous species. This may be promoted or even be enabled by surface hydroxyl groups. Hence we were investigating the interaction of CO2 and H2O with a ZrO2 model surface and the formation of different reaction intermediates (HxC-O and O-C=O species). Another possibility of CO2 activation is directly by chemical reaction. Therefore, we are studying methane dry reforming on invers Pt/ZrO2 model catalysts (i.e. ZrO2 islands on Pt single crystals) and on perovskite-type catalysts.
In our investigations of these systems, we focus on structural changes during reaction and try to identify the role of the interface between oxide and active metal, both with NAP-XPS and InfraRed spectroscopies (IR).
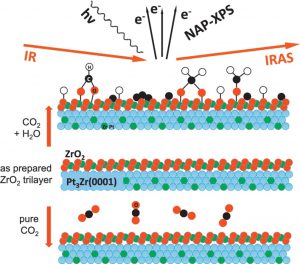
Hao Li et al., Surf. Sci., 2019
Past Projects (Examples)
- Interaction of Solid Surfaces with Humidity studied by Synchrotron based NAP-XPS (PostDoc at Lawrence Berkeley National Lab, USA)
- Design of an Microreactor for Catalytic Testing of Single Crystal based Model Catalysts
- Noble Metal/Carbon Model Catalysts: XPS, STM, and Microreactor Studies for Ethylene Hydrogenation
- Bimetallic Catalyst Materials for Methanol Steam Reforming
- Cobalt Oxide Model Catalysis Across the Materials and Pressure Gap
